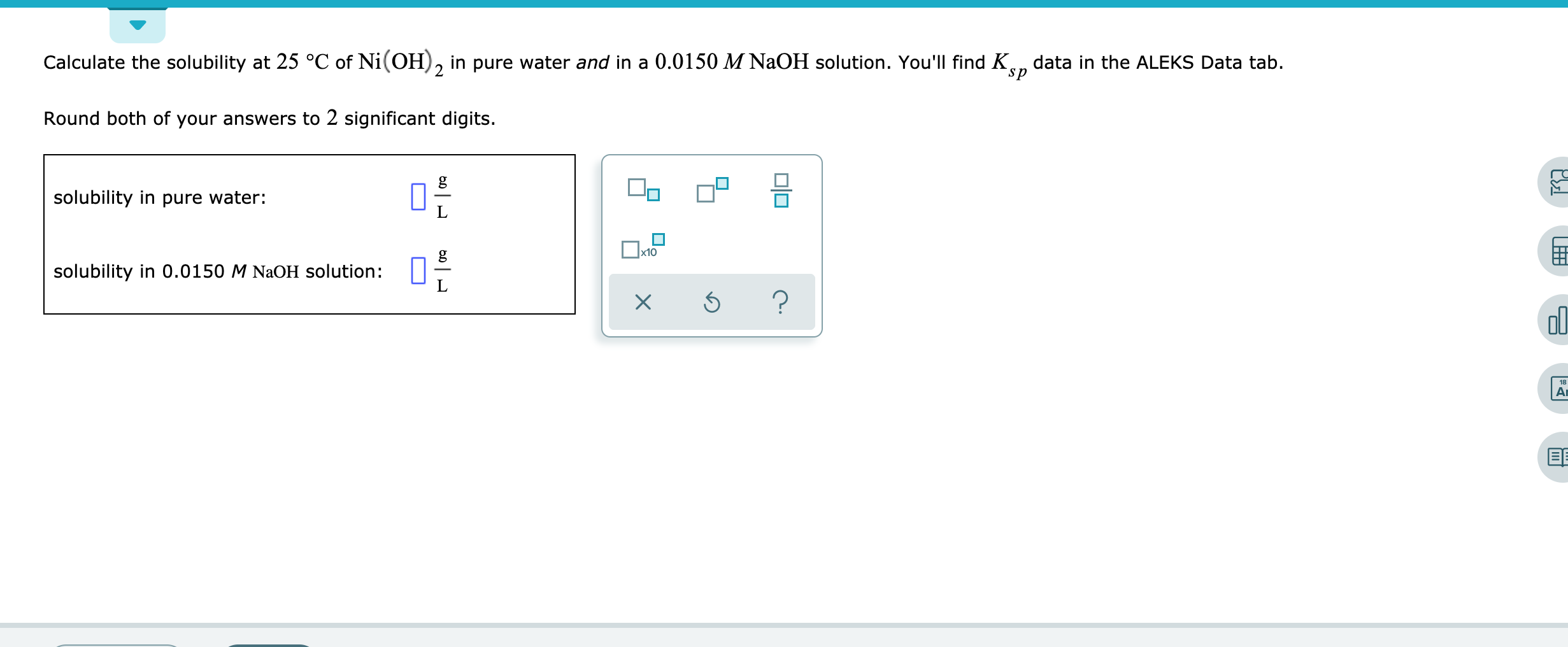
Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic product of Ni(OH)2 is..... - YouTube

Nickel hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution containing nickel ions. Nickel hydroxide (Ni(OH)2) is precipitated Stock Photo - Alamy

Find out the solubility of Ni(OH)2 in 0.1 M NaOH.Given that the ionic product of Ni(OH)2 is 2×10-15 - YouTube

Porous Fe-Doped β-Ni(OH)2 Nanopyramid Array Electrodes for Water Splitting | ACS Applied Materials & Interfaces

Calculate the molar solubility of Ni(OH)2 in 0.10 M NaOH solution. The ionic product of Ni(OH)2 2.0 × 10^-15

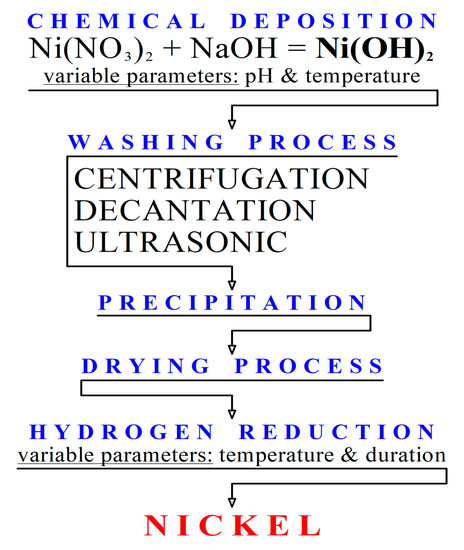
5 Six methods of preparing Ni(OH) 2. (a) Basification of a nickel(II)... | Download Scientific Diagram
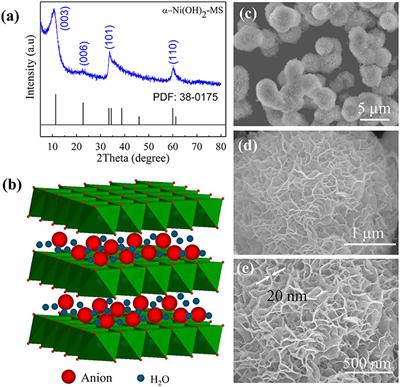
Frontiers | Facile Synthesis of Monodispersed α-Ni(OH)2 Microspheres Assembled by Ultrathin Nanosheets and Its Performance for Oxygen Evolution Reduction

Higher-Valent Nickel Oxides with Improved Oxygen Evolution Activity and Stability in Alkaline Media Prepared by High-Temperature Treatment of Ni(OH) 2 | ACS Catalysis

NEET 2020 SOLUTION -Find out the solubility of Ni(OH)2 in 0.1 M NaOH . Given that the ionic product - YouTube
Frontiers | Facile Synthesis of Monodispersed α-Ni(OH)2 Microspheres Assembled by Ultrathin Nanosheets and Its Performance for Oxygen Evolution Reduction

One material, multiple functions: graphene/Ni(OH)2 thin films applied in batteries, electrochromism and sensors | Scientific Reports

136. Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic product of Ni(OH)2 is 2.0x10-15 1) 2 x 10-13M 2) 10-12M 3) 2 x 10-'M 4) 4 x 10-13M following

Strongly Coupled Ni/Ni(OH)2 Hybrid Nanocomposites as Highly Active Bifunctional Electrocatalysts for Overall Water Splitting | ACS Sustainable Chemistry & Engineering

Figure 5 from Thermodynamic model of Ni(II) solubility, hydrolysis and complex formation with ISA | Semantic Scholar

Ionic product of Ni(OH)2is 2.0 x 10-15Molar solubility of Ni(OH)2in 0.10 M NaOH will bea)1.0 x 10-13Mb)2.0 x 10-13Mc)4.0 x 10-13Md)8.0 x 10-13MCorrect answer is option 'B'. Can you explain this answer? -

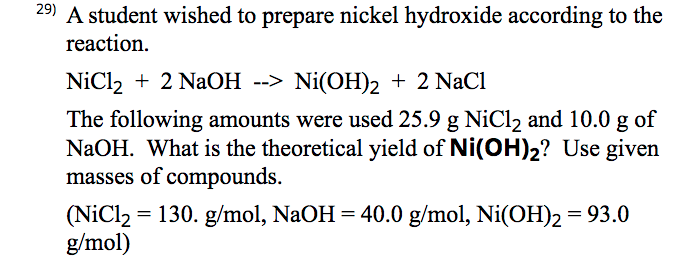



![Malayalam] Calculate the solubility of Ni(OH)2 in 0.1M NaOH solution. Malayalam] Calculate the solubility of Ni(OH)2 in 0.1M NaOH solution.](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/6531163.webp)