
The formation constant of `Ni(NH_3)_6^(2+)` is `6xx10^8` at `25^@C`.If 50 ml of 2.0 M `NH_3` is - YouTube

Table 4 from Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate | Semantic Scholar
2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub. Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.](https://cyberleninka.org/viewer_images/173973/f/1.png)
2. The... | Download Scientific Diagram Representation of the cubic structure of [Ni(NH3)6](NO3)2. The... | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig8/AS:667921089036294@1536256205990/Representation-of-the-cubic-structure-of-NiNH36NO32-The-constituent-atoms.jpg)


![Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram](https://www.researchgate.net/publication/228364596/figure/fig3/AS:667854408003587@1536240307249/Absorption-spectra-of-NiH-2-O-6-2-and-NiNH-3-6-2-in-aqueous-solution-The.png)

![Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S02.png)
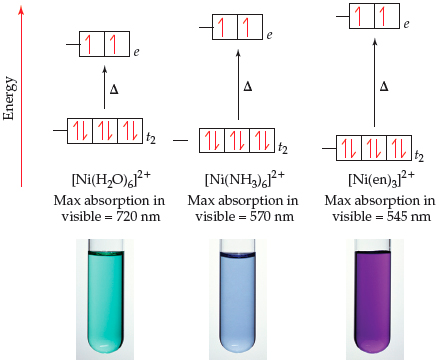
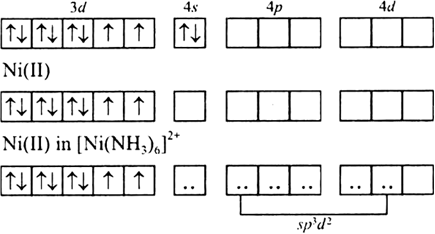
![why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE](https://preview.redd.it/why-cant-ni-nh3-6-2-have-dsp3-hybridization-then-v0-slwfvbv55ria1.png?width=640&crop=smart&auto=webp&s=f4e658df50fc25da185b4ccd9590bfb69fc4c902)
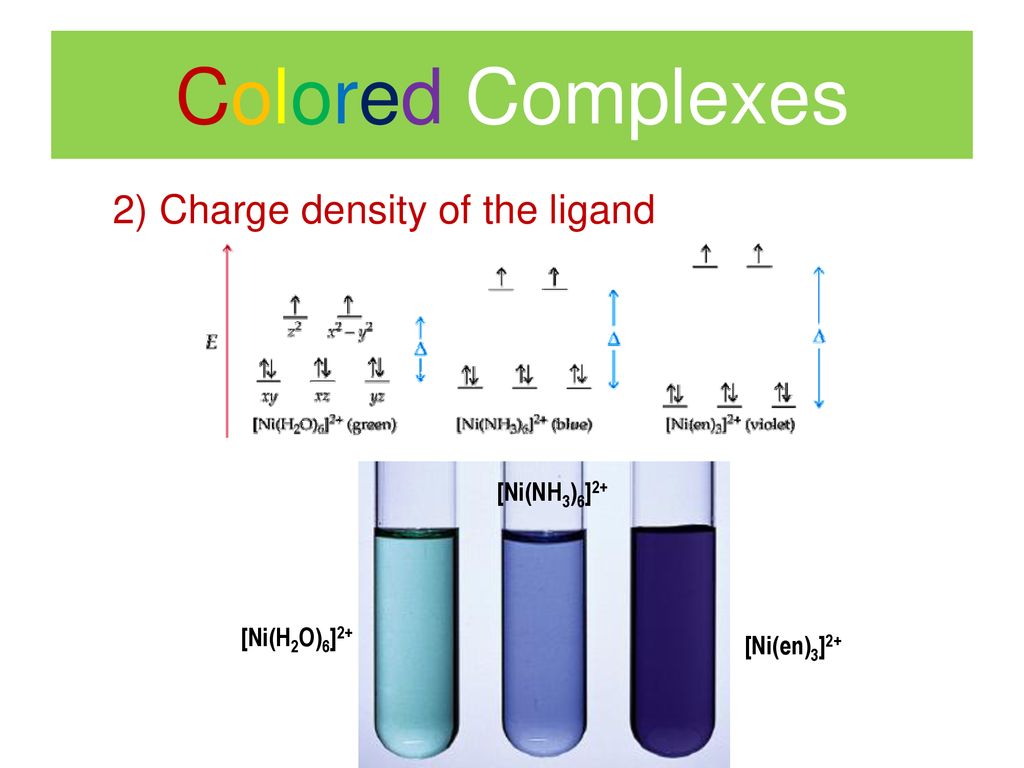
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-5256b41f5879418f6611bf7cb42000b7.webp)