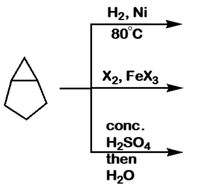
Ni+H2SO4=H2+Ni2(SO4)3 Balanced Equation||Nickel+Sulphuric acid=Hydrogen+Nickel sulphate Balanced Equ - YouTube
Characteristics of Leaching of Nickel from a Mafic Overburden in Sulfuric Acid and Sodium Chloride Medium at Atmospheric Pressure | SpringerLink

Comparison between experimental and calculated I-E curves. Cu-Ni in 0.5 M H2SO4 + 10 −4 M cysteine: cathodic scan (cf. Fig. 2).

A Little Nickel Goes a Long Way: Ni Incorporation into Rh2P for Stable Bifunctional Electrocatalytic Water Splitting in Acidic Media | ACS Materials Au
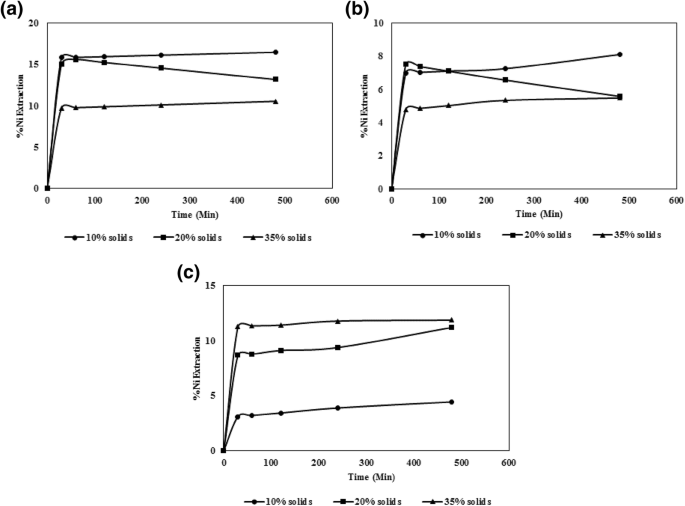
Effect of H2SO4 concentration on Ni leaching. Conditions: (a) 6 vol.%... | Download Scientific Diagram

Carbon-supported Ni(OH)2 nanospheres decorated with Au nanoparticles: a promising catalyst for BH4− electrooxidation | Ionics

Kinetics of nickel leaching from low-nickel matte in sulfuric acid solution under atmospheric pressure - ScienceDirect
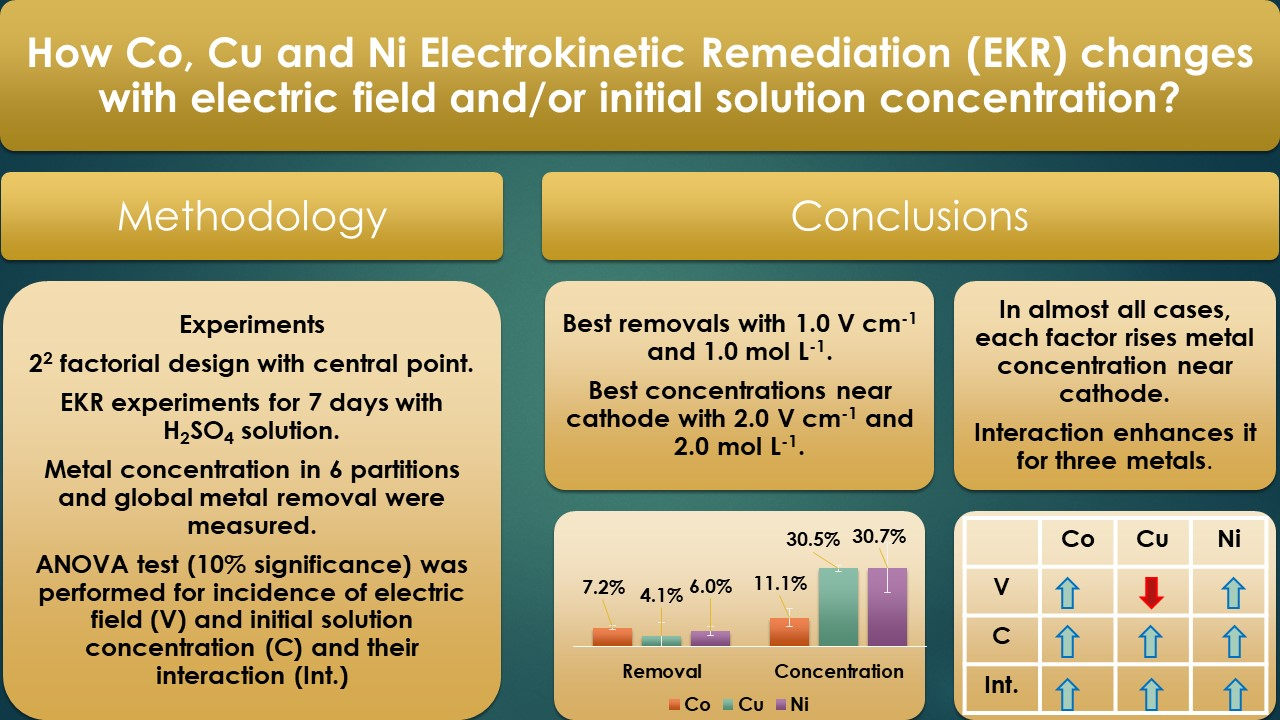
Processes | Free Full-Text | Incidence of Electric Field and Sulfuric Acid Concentration in Electrokinetic Remediation of Cobalt, Copper, and Nickel in Fresh Copper Mine Tailings

Synergistic Recovery of Valuable Metals from Spent Nickel–Metal Hydride Batteries and Lithium-Ion Batteries | ACS Sustainable Chemistry & Engineering

a LSVs for the HER on the bare Ni foam (a) and NiBTC/Ni foam (b) in... | Download Scientific Diagram

a HER polarization curves in 1 M H2SO4 for chalcogenide gel on Ni foam,... | Download Scientific Diagram

Green separation and recovery of cobalt and nickel from sulphuric acid achieved by complexation-assisted solvent extraction - ScienceDirect