Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx1.jpg)
Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
![Why a solution of [Ni(H2O)6]^2 + is green while a solution of [Ni(CN)4]^2 - is colourless? (At. no. of Ni = 28) Why a solution of [Ni(H2O)6]^2 + is green while a solution of [Ni(CN)4]^2 - is colourless? (At. no. of Ni = 28)](https://haygot.s3.amazonaws.com/questions/1194031_1237569_ans_624888e40c0e480caf24f69d44c6e352.jpg)
Why a solution of [Ni(H2O)6]^2 + is green while a solution of [Ni(CN)4]^2 - is colourless? (At. no. of Ni = 28)
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx2.jpg)
Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect
✓ Solved: Describe the distribution of d electrons in [Ni(H2O)6]^2+, using crystal field theory. How...
What is the hybridization, transition, spin, colour, magnetism, and geometry of [Ni(H2O) 4] +2? - Quora
![Jesus Jover on X: "These are the same Ni(II) compounds in the solid state as prepared by the students. The one missing is [Ni(H2O)6]SO4 that we obviously did not synthesize. @j_cirera @GamezGroup Jesus Jover on X: "These are the same Ni(II) compounds in the solid state as prepared by the students. The one missing is [Ni(H2O)6]SO4 that we obviously did not synthesize. @j_cirera @GamezGroup](https://pbs.twimg.com/media/EEsUqRBWkAAEZK2.jpg)
Jesus Jover on X: "These are the same Ni(II) compounds in the solid state as prepared by the students. The one missing is [Ni(H2O)6]SO4 that we obviously did not synthesize. @j_cirera @GamezGroup
![IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O )6](SO4)2 IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O )6](SO4)2](https://journals.iucr.org/e/issues/2020/03/00/xi2016/xi2016scheme1.gif)
IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O )6](SO4)2
2. All atoms, except the | Download Scientific Diagram Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig5/AS:667921089052683@1536256205687/Representation-of-the-unit-cell-of-NiH2O6NO32-All-atoms-except-the.jpg)
![Solved (a) The Molecular Orbital Diagram for [Ni(H2O)6]2+ is | Chegg.com Solved (a) The Molecular Orbital Diagram for [Ni(H2O)6]2+ is | Chegg.com](https://media.cheggcdn.com/study/284/28408777-e2bb-4645-8742-fab9e76a7424/image)
![a) Absorption spectrum of the [Ni(H2O)4]²⁺ complex in the spectral... | Download Scientific Diagram a) Absorption spectrum of the [Ni(H2O)4]²⁺ complex in the spectral... | Download Scientific Diagram](https://www.researchgate.net/publication/279288804/figure/fig2/AS:1132476902703108@1647014942540/a-Absorption-spectrum-of-the-NiH2O4-complex-in-the-spectral-region-395-795-nm.jpg)
![Theoretical Study of [Ni (H2O)n]2+(H2O)m (n ≤ 6, m ≤ 18) | The Journal of Physical Chemistry A Theoretical Study of [Ni (H2O)n]2+(H2O)m (n ≤ 6, m ≤ 18) | The Journal of Physical Chemistry A](https://pubs.acs.org/cms/10.1021/jp108503e/asset/images/medium/jp-2010-08503e_0015.gif)

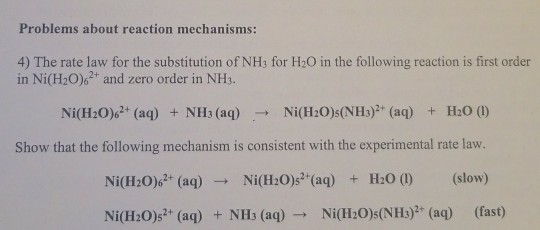
![a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram](https://www.researchgate.net/publication/279288804/figure/fig1/AS:1132476902711296@1647014942438/a-Absorption-spectrum-of-the-NiH2O6-complex-in-the-spectral-region-395-795-nm.jpg)

![Solved Part A hexaaquanickel(II) chloride 0 [Ni(H2O)4]C1 O | Chegg.com Solved Part A hexaaquanickel(II) chloride 0 [Ni(H2O)4]C1 O | Chegg.com](https://media.cheggcdn.com/study/c24/c24e28cb-e04a-4e49-8a9a-d56810e6983e/image)

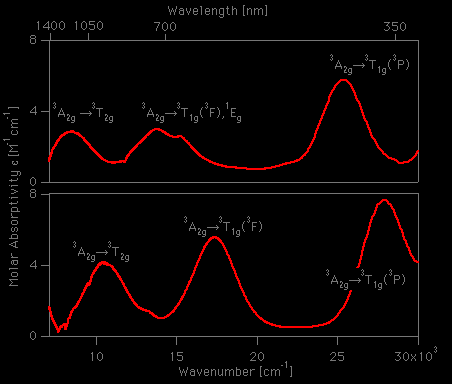

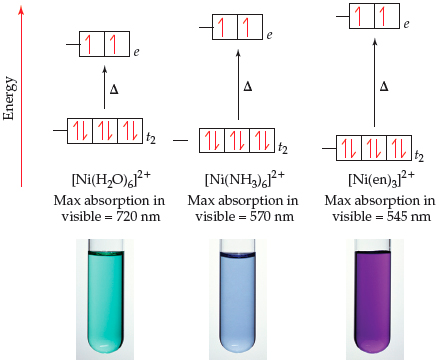
2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar FIR Spectra of [Ni(H2O)6](ClO4)2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ecaf6b0fee69dc0a5060cdb8d7e5d0c7d395a06e/4-Table1-1.png)